Chunlei Liu @ UC Berkeley
Medical Imaging, Brain Imaging and Cell Modulation
Remote Cell Modulation using Electromagnetic Fields
Pharmacologic intervention and electrophysiology are two
classical methods for modulating cell membrane permeability to
various ions. Pharmacological intervention uses chemical
compounds; electrophysiology uses minute electrodes or patch
clamps to apply electrical potential cross cell membrane. The
former lacks cellular specificity and may create many
confounding effects; the latter is difficult to apply to
multiple cells and is highly invasive. Optogenetics is a
technique that uses light to activate and suppress cell
activities via optical interaction with engineered light
sensitive channel proteins such as channelrhodopsin. While
optogenetics has proved to be an extremely powerful technique,
light does not penetrate biological tissue well. We are
developing a method that uses electromagnetic fields together
with engineering membrane proteins to modulate cell
activities. In one example, we fuse temperature sensitive
membrane proteins such as the transient receptor potential
channels (e.g. TRPV1) with a small ferritin-binding domain 5
(D5) of kininogen-1. These fusion proteins result in
endogenous Ferritin-iron Redistribution to Ion Channels
(FeRIC). These ion-channel-bound ferritins interact with
applied RF waves, which triggers calcium influx through TRPV1
channels. In one application, we used FeRIC to transiently
activate TRPV1 or TRPV4 in neural crest cells in chick embryos
to mimic fever-induced stimulation of these channels. TRPV1 or
TRPV4 activation resulted in cardiac and craniofacial birth
defects similar to those induced by fever. These results
suggest that preventing TRPV1 and TRPV4 activation during
first trimester febrile episodes may reduce the incidence of
common forms of birth defects. 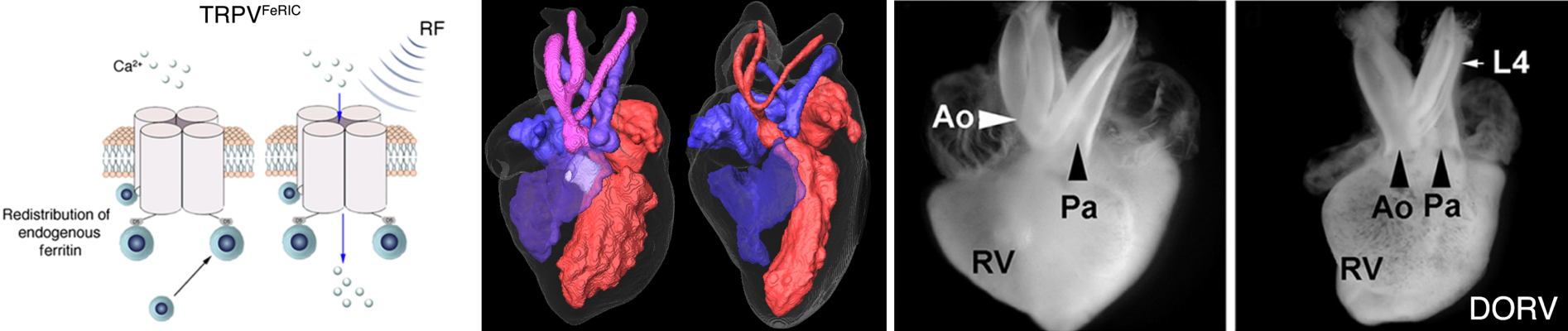
Hutson, M. R., Keyte, A. L., Hernández-Morales, M., Gibbs, et al. (2017). Temperature-activated ion channels in neural crest cells confer maternal fever–associated birth defects. Sci. Signal., 10(500), eaal4055.
CNN, Oct 10, 2017: How fever in early pregnancy can cause birth defects.
Hernández-Morales, M., Shang, T., Chen, J., Han, V., & Liu, C. (2020). Lipid Oxidation Induced by RF Waves and Mediated by Ferritin Iron Causes Activation of Ferritin-Tagged Ion Channels. Cell reports, 30(10), 3250-3260.
Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) is a non-invasive technology for imaging human body. It is used daily in hospitals for aiding medical diagnosis; it is also widely used for studying human brain structure and function. Our lab develops methods to improve existing MRI techniques or create new forms of MRI for improving medical diagnosis and our understanding of human brain. We are broadly interested in all things related to MRI. The following are a few examples of projects we are working on.
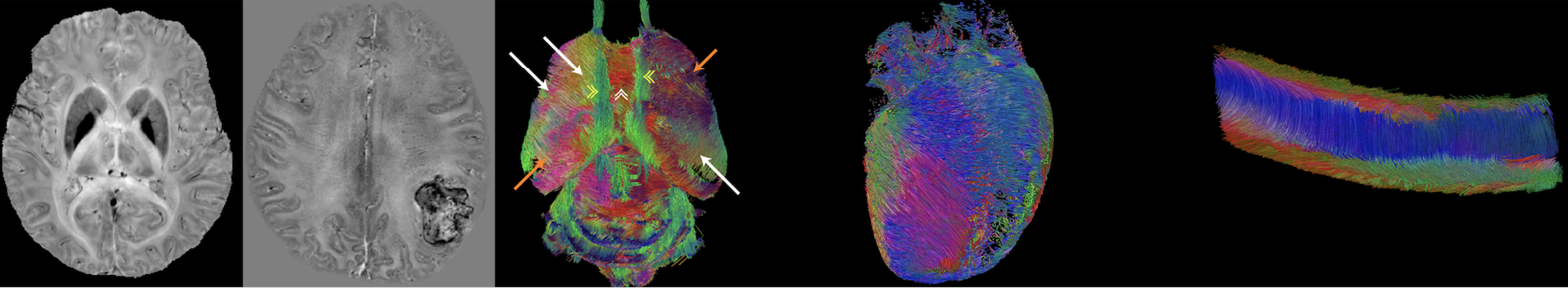
Imaging and quantifying magnetic susceptibility
Magnetic susceptibility is a physical quantity that defines
how strongly a material can be magnetized by an applied
magnetic field. While classical magnetometers or SQUID
detectors can measure bulk magnetic susceptibility, they do
not measure the anatomical distribution of magnetic
susceptibility inside the human body. We are developing
MRI-based methods to measure the spatial distribution of
magnetic susceptibility in biological tissues. This is
possible because magnetic susceptibility changes the spatial
pattern of magnetic field which in turn changes the
frequency of MRI signal. These methods are now generally
called quantitative susceptibility mapping (QSM) and
susceptibility tensor imaging (STI). QSM and STI use the
phase information of gradient echo MRI to produce
high-resolution 3D maps of magnetic susceptibility which
reflect the local molecular contents and tissue
architecture. QSM and STI have been used to quantify tissue
iron stores, calcification, myelination in white matter and
the dynamic conversion between oxy- and deoxyhemoglobin. By
quantifying magnetic susceptibility anisotropy, STI allows
the mapping of the orientations of axonal fiber, myofiber
and collagen.
Liu, C., Li, W., Tong, K. A., Yeom, K. W., & Kuzminski,
S. (2015). Susceptibility‐weighted
imaging and quantitative susceptibility mapping in the
brain. Journal of magnetic resonance imaging, 42(1),
23-41.
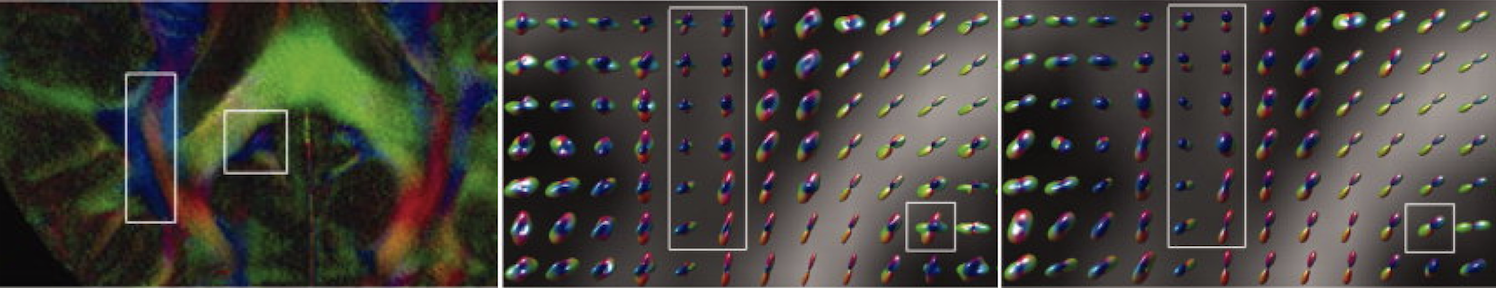
Imaging and quantifying molecular diffusion
MRI is the only technique that can image and quantify
molecular diffusion inside the human body non-invasively.
Knowing the properties of molecular diffusion can tell us
information about tissue microstructure as molecular
movement is affected by various biological membranes and
cell density. We are developing image acquisition and
reconstruction techniques to generate high quality
diffusion-weighted images. We are also developing
mathematical models to relate diffusion-weighted MRI signals
to the underlying tissue properties. For example, we have
pioneered the method to use higher order tensors to
characterize non-Gaussian diffusion observed in biological
tissues. These higher order tensors include covariance
tensor (2nd order), skewness tensor (3rd order), kurtosis
tensor (4th order) and so on.
Liu, C., et al. Generalized
diffusion tensor imaging (GDTI) using higher-order tensor
(HOT) statistics. 11th ISMRM, Toronto, 2003. p 242
Liu, C., Bammer, R., & Moseley, M. E. (2003). Generalized
Diffusion Tensor Imaging (GDTI): A Method for
Characterizing and Imaging Diffusion Anisotropy Caused by
Non‐Gaussian Diffusion. Israel Journal of Chemistry,
43(1‐2), 145-154.
Liu, C., Bammer, R., Acar, B., & Moseley, M. E. (2004).
Characterizing
non‐Gaussian diffusion by using generalized diffusion
tensors. Magnetic Resonance in Medicine, 51(5),
924-937.
Ultra-high field MRI
We are involved in a project funded by NIH BRAIN Initiative
to develop a next-generation human brain MRI scanner that
utilizes 7-Tesla magnetic field.
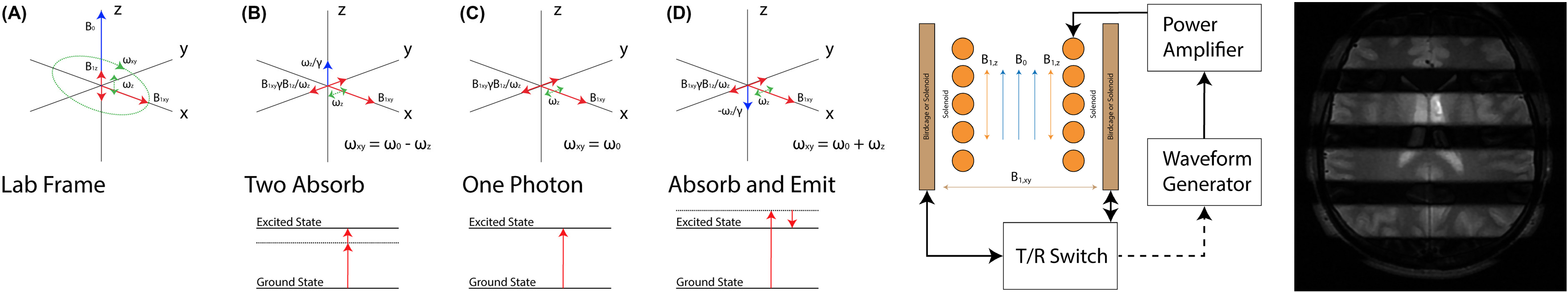
Multiphoton MRI
Today’s MRI assumes single‐photon excitation.1 That is, for
each nuclear spin, a single photon accompanies the
transition between energy states. This photon must resonate
near the Larmor frequency. Here, we show that, instead of
the usual single‐photon resonance, we can excite multiphoton
resonances to generate signal for MRI by using multiple
magnetic field frequencies, none of which is near the Larmor
frequency. Only the total energy absorbed by a spin must
correspond to the Larmor frequency.
Han, Victor, and Chunlei Liu. "Multiphoton magnetic resonance in imaging: A classical description and implementation." Magnetic Resonance in Medicine (2020).
Victor Han, Finalist for 2020 ISMRM I.I. Rabi Award for work in multiphoton MRI.
Neurodegeneration - Parkinson's and Alzheimer's
Neurodegeneration refers to the progressive atrophy and loss
of function of neurons, which is present in neurodegenerative
diseases such as Alzheimer's disease and Parkinson's disease.
The technologies we develop have been applied to improve the
understanding, diagnosis and treatment of these diseases. For
example, the susceptibility MRI techniques are used for
studying aggregation of pathological proteins and detecting
structural and functional changes, and for deep brain
stimulation surgical planning. 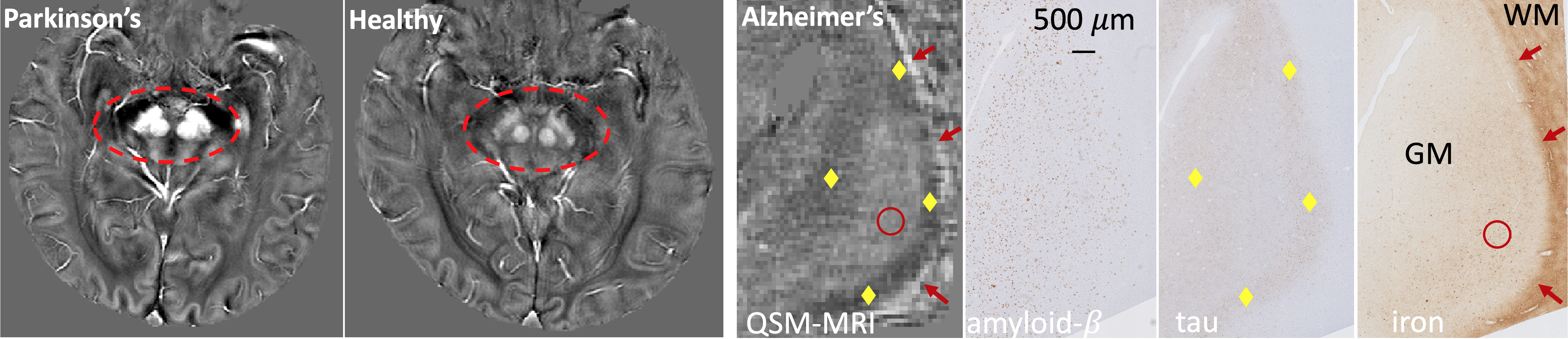
Guan, X., Xuan, M., Gu, Q., Huang, P., Liu, C., Wang, N., ... & Zhang, M. (2017). Regionally progressive accumulation of iron in Parkinson's disease as measured by quantitative susceptibility mapping. NMR in Biomedicine, 30(4), e3489.
He, N., Huang, P., Ling, H., Langley, J., Liu, C., Ding, B., ... & Hu, X. (2017). Dentate nucleus iron deposition is a potential biomarker for tremor‐dominant Parkinson's disease. NMR in Biomedicine, 30(4), e3554.
Guan, X., Huang, P., Zeng, Q., Liu, C., Wei, H., Xuan, M., ... & Luo, X. (2019). Quantitative susceptibility mapping as a biomarker for evaluating white matter alterations in Parkinson’s disease. Brain imaging and behavior, 13(1), 220-231.
He, N., Ling, H., Ding, B., Huang, J., Zhang, Y., Zhang, Z., ... & Yan, F. (2015). Region‐specific disturbed iron distribution in early idiopathic P arkinson's disease measured by quantitative susceptibility mapping. Human brain mapping, 36(11), 4407-4420.
Guan, X., Zhang, Y., Wei, H., Guo, T., Zeng, Q., Zhou, C., ... & Xu, X. (2019). Iron-related nigral degeneration influences functional topology mediated by striatal dysfunction in Parkinson's disease. Neurobiology of aging, 75, 83-97.
Guan, X., Guo, T., Zhou, C., Wu, J., Gao, T., Bai, X., ... & Huang, P. (2020). Asymmetrical nigral iron accumulation in Parkinson’s disease with motor asymmetry: an explorative, longitudinal and test-retest study. Aging (Albany NY), 12(18), 18622.
Gong, N. J., Dibb, R., Bulk, M., van der Weerd, L., & Liu, C. (2019). Imaging beta amyloid aggregation and iron accumulation in Alzheimer's disease using quantitative susceptibility mapping MRI. Neuroimage, 191, 176-185.
Gong, N. J., Chan, C. C., Leung, L. M., Wong, C. S., Dibb, R., & Liu, C. (2017). Differential microstructural and morphological abnormalities in mild cognitive impairment and A lzheimer's disease: Evidence from cortical and deep gray matter. Human brain mapping, 38(5), 2495-2508.
Li, W., Langkammer, C., Chou, Y. H., Petrovic, K., Schmidt, R., Song, A. W., ... & Liu, C. (2015). Association between increased magnetic susceptibility of deep gray matter nuclei and decreased motor function in healthy adults. Neuroimage, 105, 45-52.
Wei, H., Zhang, C., Wang, T., He, N., Li, D., Zhang, Y., ... & Sun, B. (2019). Precise targeting of the globus pallidus internus with quantitative susceptibility mapping for deep brain stimulation surgery. Journal of Neurosurgery, 1(aop), 1-7.
Li, J., Li, Y., Gutierrez, L., Xu, W., Wu, Y., Liu, C., ... & Wei, H. (2020). Imaging the Centromedian Thalamic Nucleus Using Quantitative Susceptibility Mapping. Frontiers in Human Neuroscience, 13, 447.
Guan, X., Guo, T., Zeng, Q., Wang, J., Zhou, C., Liu, C., ... & Xu, X. (2019). Oscillation-specific nodal alterations in early to middle stages Parkinson’s disease. Translational Neurodegeneration, 8(1), 36.